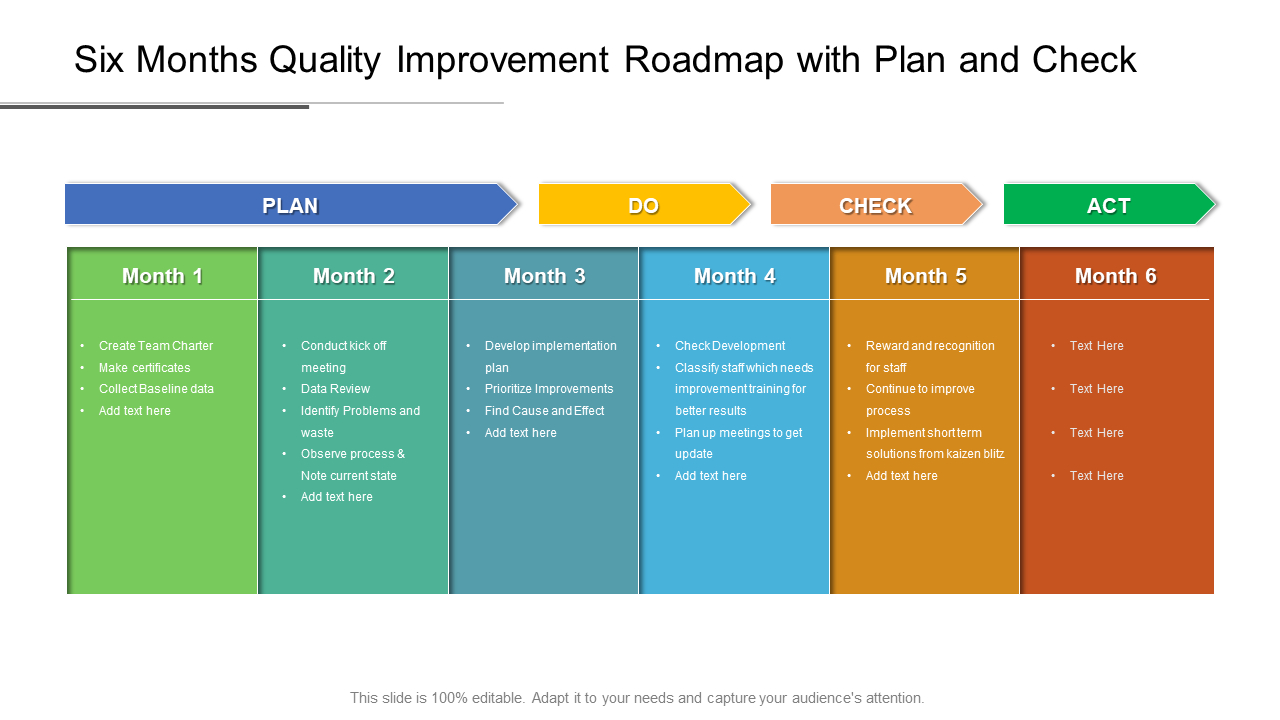
Sample Of Medical Device Quality Plan Template
Sample Of Medical Device Quality Plan Template - The document should be tailored to the specific requirements based on the. Download them now for free as word, pdf,. The iso 13485 is the standard for quality management in the medical device industry. In this article, we will cover the iso 13485 and fda requirements for a quality policy, and provide examples of quality policies from various medical device companies. If a patient experiences chronic tonsil stone. Here is a sample from a quality plan written for a hypothetical device that deals with design verification: A letter of medical necessity template is often used to justify the need for specific medical procedures, such as tonsil stone removal. Documentation to comply with mdr and iso 13485 (medical device), iso 27001 (cybersecurity), iso 9001 (quality), iso 14001 (environmental), iso 45001 (health & safety), nis 2 (critical. Complete iso 13485 and fda qsr compliant quality system templates for medical device businesses. Here are all our posts on this standard, and also all questions our consulting clients. Covering iso 13485, iec 62304, iso 14971 and iec 62366. Complete iso 13485 and fda qsr compliant quality system templates for medical device businesses. Here is a sample from a quality plan written for a hypothetical device that deals with design verification: Let us help you focus on. The document should be tailored to the specific requirements based on the. A letter of medical necessity template is often used to justify the need for specific medical procedures, such as tonsil stone removal. This medical devices development plan describes in detail all essential steps to be considered prior to start the development of medical devices and is in alignment with current fda and. The iso 13485 is the standard for quality management in the medical device industry. If a patient experiences chronic tonsil stone. Here are all our posts on this standard, and also all questions our consulting clients. Complete iso 13485 and fda qsr compliant quality system templates for medical device businesses. Let us help you focus on. The document should be tailored to the specific requirements based on the. This document is intended to form the basis for a supplier agreement for a medical device manufacturer. A letter of medical necessity template is often used to justify. A letter of medical necessity template is often used to justify the need for specific medical procedures, such as tonsil stone removal. Here is a sample from a quality plan written for a hypothetical device that deals with design verification: Complete iso 13485 and fda qsr compliant quality system templates for medical device businesses. Here are all our posts on. Each manufacturer shall establish a quality plan which defines the quality practices, resources, and activities relevant to devices that are designed and manufactured. Documentation to comply with mdr and iso 13485 (medical device), iso 27001 (cybersecurity), iso 9001 (quality), iso 14001 (environmental), iso 45001 (health & safety), nis 2 (critical. Medqdoc provides 28 templates to support you in compiling the. Documentation to comply with mdr and iso 13485 (medical device), iso 27001 (cybersecurity), iso 9001 (quality), iso 14001 (environmental), iso 45001 (health & safety), nis 2 (critical. Here is a sample from a quality plan written for a hypothetical device that deals with design verification: Medqdoc provides 28 templates to support you in compiling the correct technical documentation for your. The medqdoc configuration is built. Covering iso 13485, iec 62304, iso 14971 and iec 62366. If a patient experiences chronic tonsil stone. Documentation to comply with mdr and iso 13485 (medical device), iso 27001 (cybersecurity), iso 9001 (quality), iso 14001 (environmental), iso 45001 (health & safety), nis 2 (critical. Let us help you focus on. Let us help you focus on. Download them now for free as word, pdf,. This medical devices development plan describes in detail all essential steps to be considered prior to start the development of medical devices and is in alignment with current fda and. The document should be tailored to the specific requirements based on the. In this article, you. Medqdoc provides 28 templates to support you in compiling the correct technical documentation for your medical device, to meet regulatory requirements. A letter of medical necessity template is often used to justify the need for specific medical procedures, such as tonsil stone removal. Med dev qms provides iso 13485:2016 and fda qsr compliant quality system templates specifically developed for startup. The iso 13485 is the standard for quality management in the medical device industry. The medqdoc configuration is built. Each manufacturer shall establish a quality plan which defines the quality practices, resources, and activities relevant to devices that are designed and manufactured. Here is a sample from a quality plan written for a hypothetical device that deals with design verification:. The medqdoc configuration is built. Medqdoc provides 28 templates to support you in compiling the correct technical documentation for your medical device, to meet regulatory requirements. The iso 13485 is the standard for quality management in the medical device industry. Here is a sample from a quality plan written for a hypothetical device that deals with design verification: A letter. If a patient experiences chronic tonsil stone. Med dev qms provides iso 13485:2016 and fda qsr compliant quality system templates specifically developed for startup & small medical devices firms. Design verification shall be performed in accordance with sopx.1234. Medqdoc provides 28 templates to support you in compiling the correct technical documentation for your medical device, to meet regulatory requirements. The. Download them now for free as word, pdf,. Documentation to comply with mdr and iso 13485 (medical device), iso 27001 (cybersecurity), iso 9001 (quality), iso 14001 (environmental), iso 45001 (health & safety), nis 2 (critical. Each manufacturer shall establish a quality plan which defines the quality practices, resources, and activities relevant to devices that are designed and manufactured. In this article, you will find a quality manual template conforming to the requirements of regulation 2017/745 and en iso 13485:2016 + a11:2021. The medqdoc configuration is built. Additionally, we’ve also got templates for the mdr clinical. Here is a sample from a quality plan written for a hypothetical device that deals with design verification: A letter of medical necessity template is often used to justify the need for specific medical procedures, such as tonsil stone removal. Complete iso 13485 and fda qsr compliant quality system templates for medical device businesses. Covering iso 13485, iec 62304, iso 14971 and iec 62366. The document should be tailored to the specific requirements based on the. Design verification shall be performed in accordance with sopx.1234. This document is intended to form the basis for a supplier agreement for a medical device manufacturer. Med dev qms provides iso 13485:2016 and fda qsr compliant quality system templates specifically developed for startup & small medical devices firms. Medqdoc provides 28 templates to support you in compiling the correct technical documentation for your medical device, to meet regulatory requirements. If a patient experiences chronic tonsil stone.Medical Device Quality Plan Template Sample Template Samples
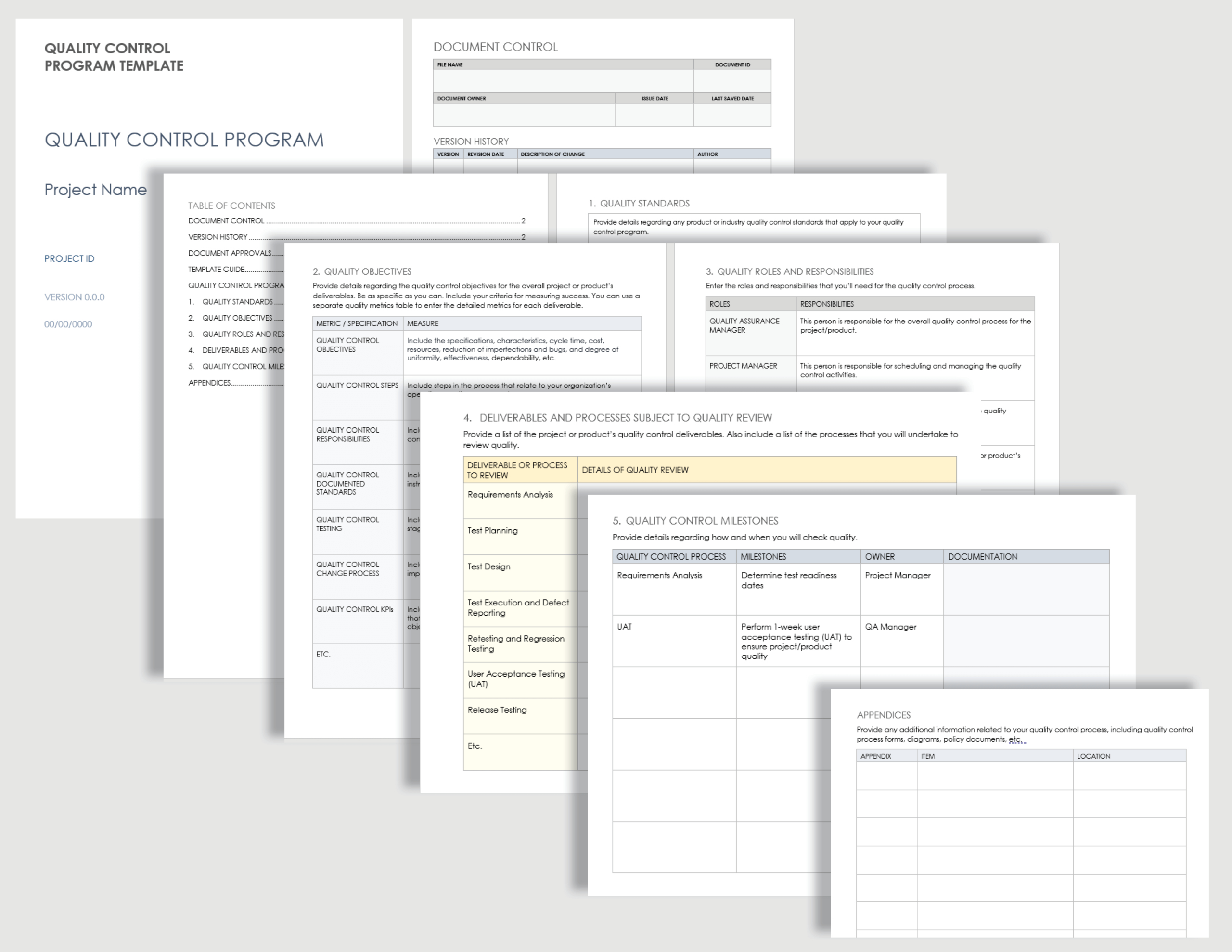
Medical Device Development Plan Template in Word, Pages, Google Docs
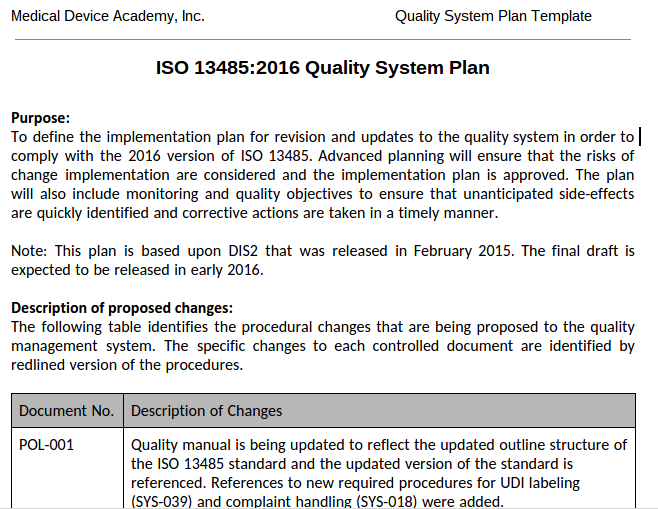
How to write a quality system plan template (free download)
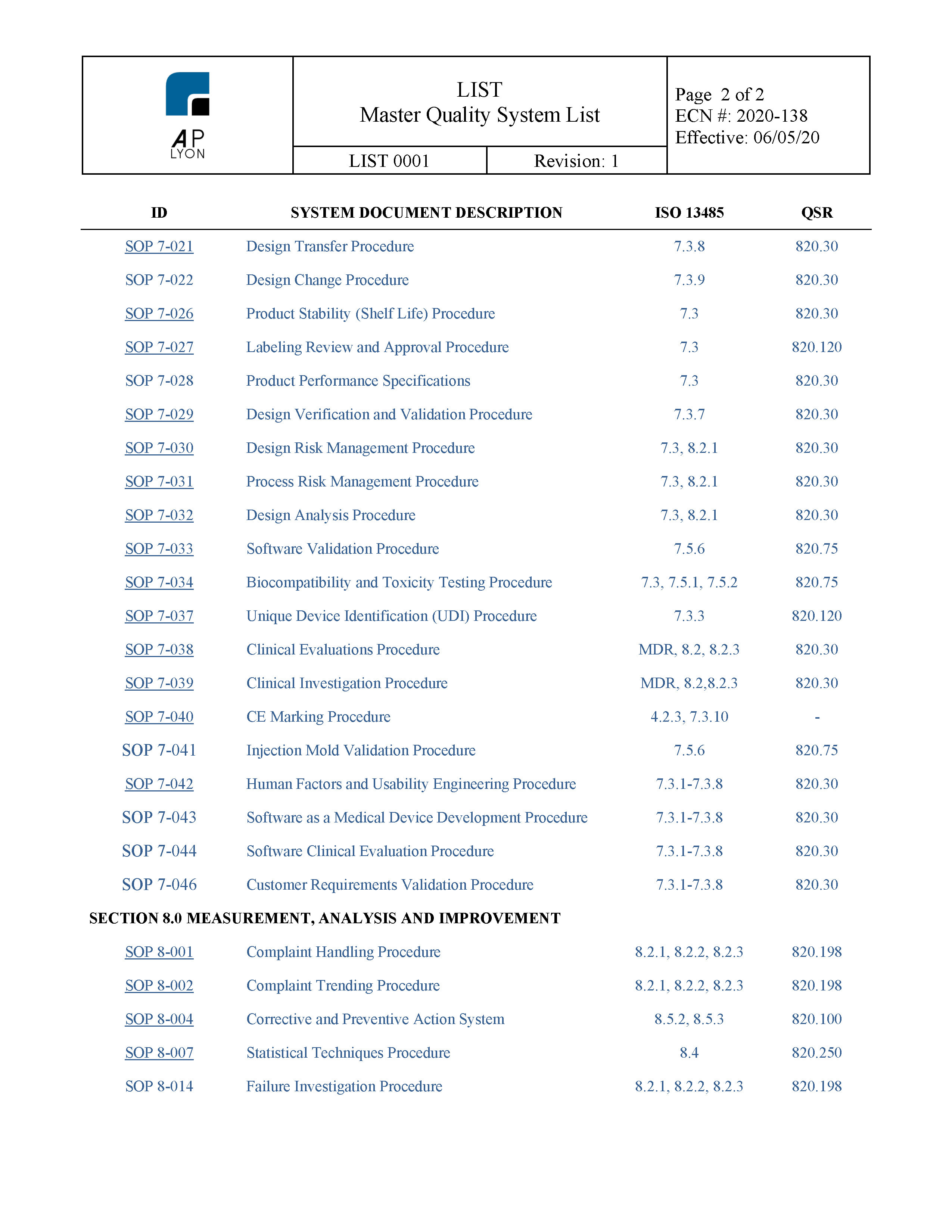
Medical device quality management system template 8 powerful options
Medical Device Quality Plan Template
Sample Of Medical Device Quality Plan Template
Medical Device Quality Plan Template Sample Template Samples
Medical Device Quality Plan Template
Medical Device Quality Plan Template Sample Template Samples
Medical Device Quality Plan Template
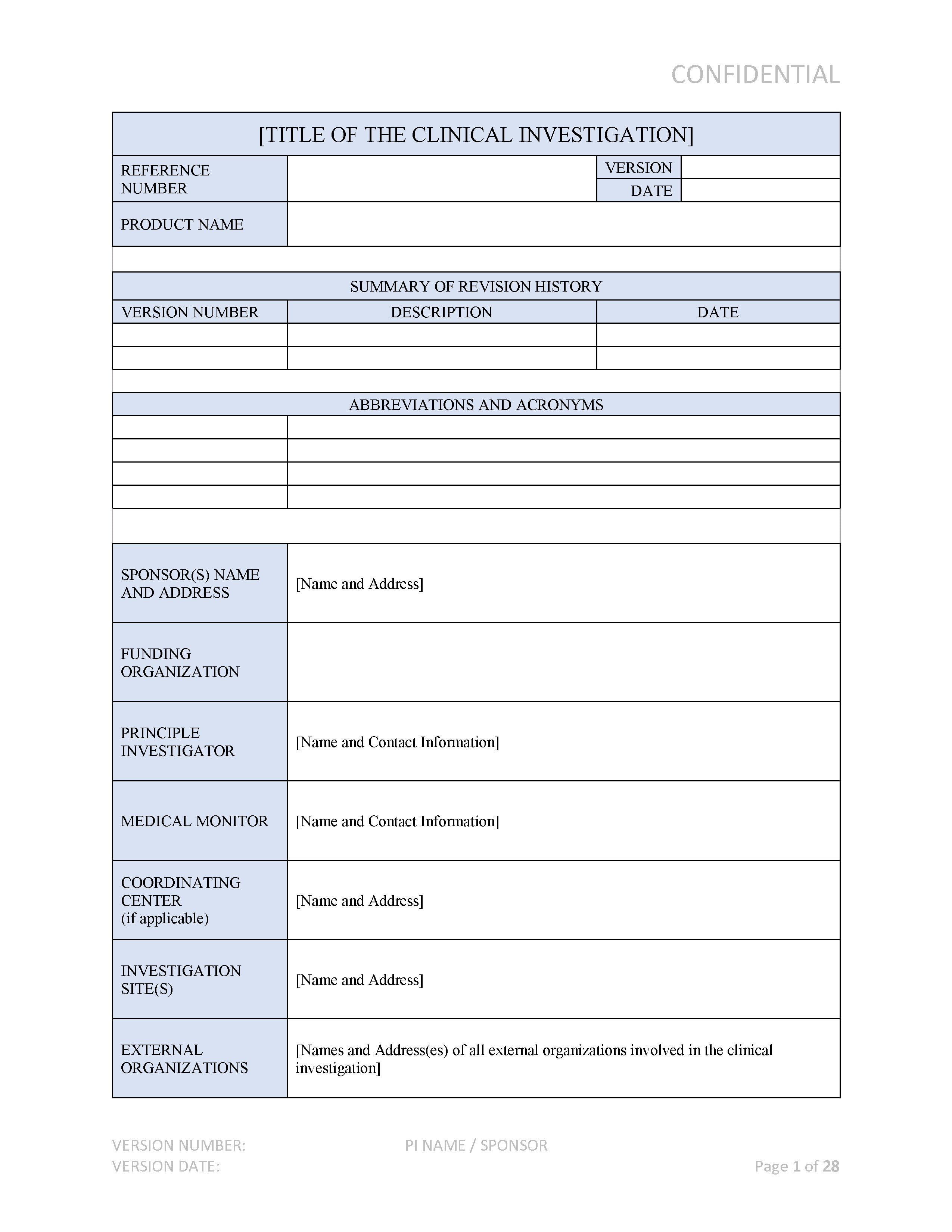
This Medical Devices Development Plan Describes In Detail All Essential Steps To Be Considered Prior To Start The Development Of Medical Devices And Is In Alignment With Current Fda And.
In This Article, We Will Cover The Iso 13485 And Fda Requirements For A Quality Policy, And Provide Examples Of Quality Policies From Various Medical Device Companies.
The Iso 13485 Is The Standard For Quality Management In The Medical Device Industry.
Here Are All Our Posts On This Standard, And Also All Questions Our Consulting Clients.
Related Post: