Other Support Template Nih
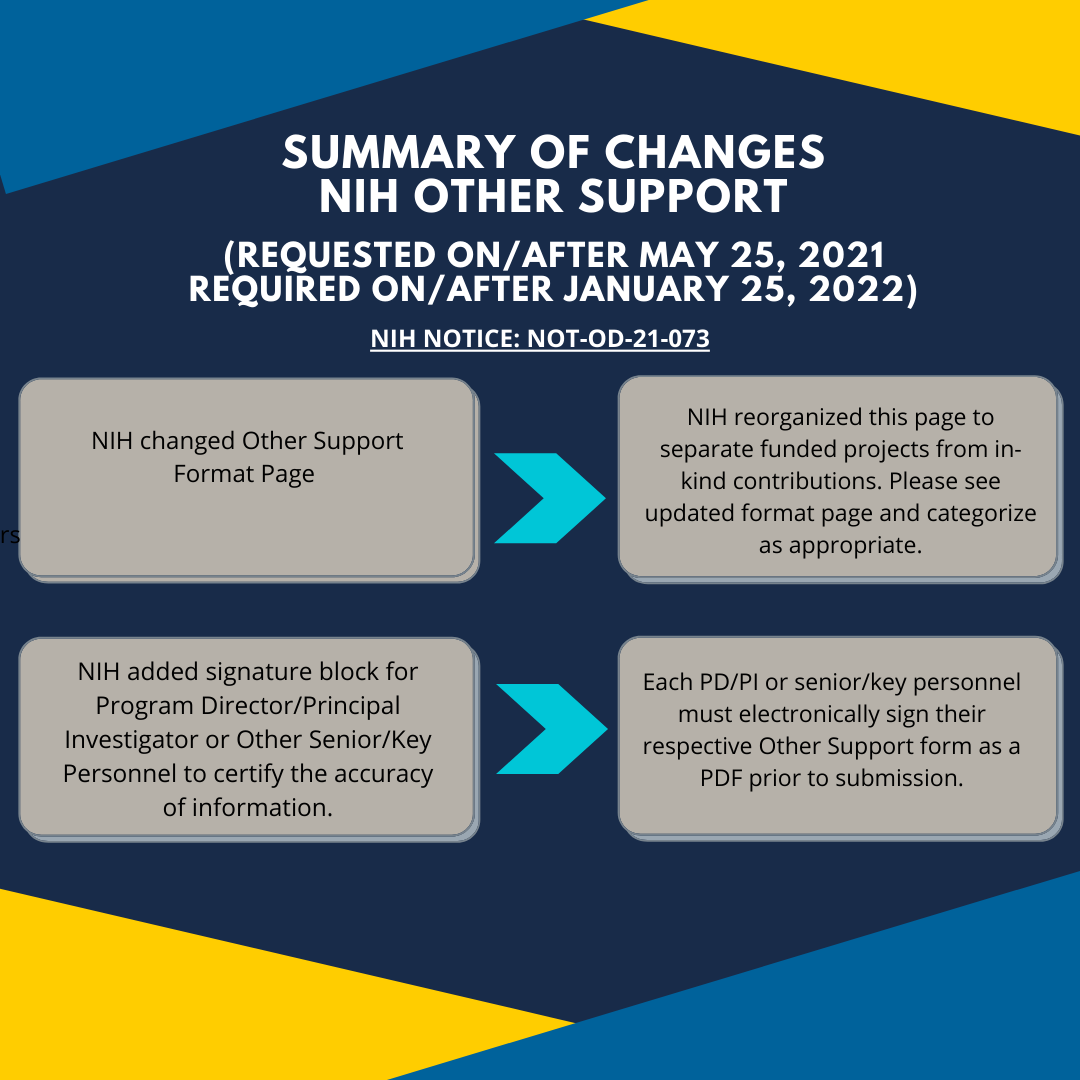
Other Support Template Nih - Application format pages for biographical sketches, other support, fellowship and training data tables, data management and sharing (dms) plans, reference. 10/2021 approved through 09/30/2024) phs other support. Nih issued a new other support form (rev. In april 2024, the office of management and budget issued revisions to the uniform administrative requirements for federal financial assistance, located at 2. As the largest public funder of biomedical research in the world, nih supports a variety of programs from grants and contracts to loan repayment. This page focuses on the new format for other support. • revised other support format. Information on nih other support. A new section on group or cluster regression discontinuity designs provides background information, key references, a sample size calculator, and webinars about using. “other support” is sometimes referred to as “current and pending support” or “active and pending support.” find instructions, blank format pages, and sample other support documents below. “other support” is sometimes referred to as “current and pending support” or “active and pending support.” find instructions, blank format pages, and sample other support documents below. As of january 25, 2022, you are required to use the new other support format page. This is an interim process while the agency works to establish sciencv for. Conformance with instructions for proposal preparation 1. 10/2021 approved through 09/30/2024) phs other support. Application format pages for biographical sketches, other support, fellowship and training data tables, data management and sharing (dms) plans, reference. 10/2021) effective as of january 25, 2022. Include the principal investigator's name at the top and number. • revised other support format. Deviations from nsf proposal preparation and submission requirements. Nih issued a new other support form (rev. Deviations from nsf proposal preparation and submission requirements. The new format for other support applies to all submissions, including. 10/2021) effective as of january 25, 2022. Information on other support should be provided in the format shown below, using continuation pages as necessary. Application format pages for biographical sketches, other support, fellowship and training data tables, data management and sharing (dms) plans, reference. This page focuses on the new format for other support. As of january 25, 2022, you are required to use the new other support format page. Unless specified in a program solicitation, all. “other support” is sometimes referred to as. This page focuses on the new format for other support. The new format for other support applies to all submissions, including. Include the principal investigator's name at the top and number. “other support” is sometimes referred to as “current and pending support” or “active and pending support.” find instructions, blank format pages, and sample other support documents below. A new. • revised other support format. In accordance with the nih updated requirements for principal investigators, the other support report will include content and format changes for project and proposal information and is. Stanford faculty with a joint appointment at the va palo alto health care system (vapahcs) should use the template below for preparing their nih other support. Conformance with. Nih issued a new other support form (rev. 10/2021) effective as of january 25, 2022. • revised other support format. Deviations from nsf proposal preparation and submission requirements. “other support” is sometimes referred to as “current and pending support” or “active and pending support.” find instructions, blank format pages, and sample other support documents below. This page focuses on the new format for other support. 10/2021 approved through 09/30/2024) phs other support. In accordance with the nih updated requirements for principal investigators, the other support report will include content and format changes for project and proposal information and is. Unless specified in a program solicitation, all. In april 2024, the office of management and budget. A new section on group or cluster regression discontinuity designs provides background information, key references, a sample size calculator, and webinars about using. Nih issued a new other support form (rev. This is an interim process while the agency works to establish sciencv for. Te of health (nih) changed the way they request senior/key personnel to report other support. In. Unless specified in a program solicitation, all. Deviations from nsf proposal preparation and submission requirements. Nih issued a new other support form (rev. “other support” is sometimes referred to as “current and pending support” or “active and pending support.” find instructions, blank format pages, and sample other support documents below. This is an interim process while the agency works to. The new format for other support applies to all submissions, including. Include the principal investigator's name at the top and number. Application format pages for biographical sketches, other support, fellowship and training data tables, data management and sharing (dms) plans, reference. Deviations from nsf proposal preparation and submission requirements. In accordance with the nih updated requirements for principal investigators, the. “other support” is sometimes referred to as “current and pending support” or “active and pending support.” find instructions, blank format pages, and sample other support documents below. The new format for other support applies to all submissions, including. In april 2024, the office of management and budget issued revisions to the uniform administrative requirements for federal financial assistance, located at. As the largest public funder of biomedical research in the world, nih supports a variety of programs from grants and contracts to loan repayment. “other support” is sometimes referred to as “current and pending support” or “active and pending support.” find instructions, blank format pages, and sample other support documents below. Unless specified in a program solicitation, all. “other support” is sometimes referred to as “current and pending support” or “active and pending support.” find instructions, blank format pages, and sample other support documents below. Application format pages for biographical sketches, other support, fellowship and training data tables, data management and sharing (dms) plans, reference. Information on other support should be provided in the format shown below, using continuation pages as necessary. This page focuses on the new format for other support. Te of health (nih) changed the way they request senior/key personnel to report other support. This is an interim process while the agency works to establish sciencv for. A new section on group or cluster regression discontinuity designs provides background information, key references, a sample size calculator, and webinars about using. Include the principal investigator's name at the top and number. Stanford faculty with a joint appointment at the va palo alto health care system (vapahcs) should use the template below for preparing their nih other support. Nih issued a new other support form (rev. As of january 25, 2022, you are required to use the new other support format page. • revised other support format. Conformance with instructions for proposal preparation 1.Fillable Online Letter of Support for NIH Grant re Institutional
09250002, PHS 2590/RPPR, Other Support Format Page
PHS 398/2590, Other Support Format Page
Other Support
PPT National Institutes of Health “JustInTime” Procedures
Nih Letter Of Support Template
PPT Other support information PowerPoint Presentation, free download
NIH Other Support Signatures Sponsored Programs Office
Nih Facilities And Other Resources Template
PPT National Institutes of Health “JustInTime” Procedures
In April 2024, The Office Of Management And Budget Issued Revisions To The Uniform Administrative Requirements For Federal Financial Assistance, Located At 2.
Deviations From Nsf Proposal Preparation And Submission Requirements.
The New Format For Other Support Applies To All Submissions, Including.
10/2021) Effective As Of January 25, 2022.
Related Post: