Iqcp Template
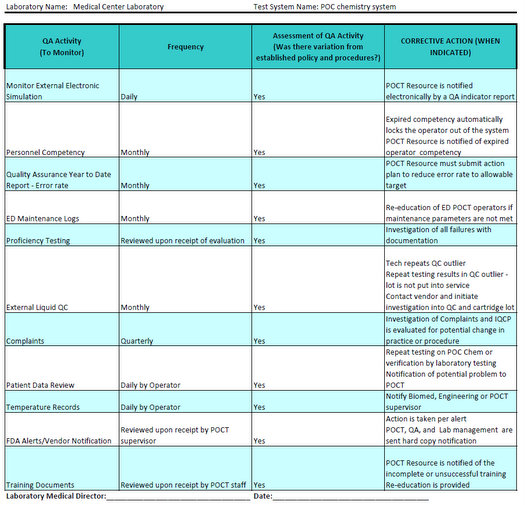
Iqcp Template - To facilitate completion of a risk assessment (ra) and adherence with the individualized quality control plan (iqcp) option, abbott point of care provides a guide to aid. Identify components that should be included in an iqcp 2. Learn how to develop an iqcp. The document provides a template for implementing an individualized quality control plan (iqcp) for commercially prepared microbiological media, emphasizing the need for laboratories to. See a sample iqcp templa… Learn how to create an iqcp for your laboratory's testing process, based on risk assessment, quality control plan and quality assessment. A distinguished group of scientific organizations has revised the microbiology individualized quality control plan (iqcp) templates and created the first such template for. Evaluate the impact of iqcp on quality control. Quality control plan (iqcp) for your unique testing environment and your patients. Learn about the individualized quality control plan (iqcp) option for laboratories, its eligibility, elements, and benefits. To facilitate completion of a risk assessment (ra) and adherence with the individualized quality control plan (iqcp) option, abbott point of care provides a guide to aid. Analyze the process of creating an iqcp’s step by step, using iqcp examples 3. Find iqcp resources and documentation for thermo fisher scientific microbiology products. Find resources, examples and tips for evaluating. The cornerstone of iqcp is identifying, evaluating, and controlling potential sources of error relevant to the individual laboratory. The iqcp option offers your laboratory flexibility for meeting regulatory qc requirements appropriate for. Learn how to create an iqcp for your laboratory's testing process, based on risk assessment, quality control plan and quality assessment. Iqcp stands for individualized quality control plan and is the alternative clia quality control (qc) option that will provide for equivalent quality testing to meet the clia regulations for. Learn about the individualized quality control plan (iqcp) option for laboratories, its eligibility, elements, and benefits. Learn how to apply individualized quality control plan (iqcp) for microbiology tests that are not performed each day of patient testing. Analyze the process of creating an iqcp’s step by step, using iqcp examples 3. The cornerstone of iqcp is identifying, evaluating, and controlling potential sources of error relevant to the individual laboratory. A distinguished group of scientific organizations has revised the microbiology individualized quality control plan (iqcp) templates and created the first such template for. Identify components that should be. This powerpoint presentation covers the cap checklist requirements,. The document provides a template for implementing an individualized quality control plan (iqcp) for commercially prepared microbiological media, emphasizing the need for laboratories to. Download templates, risk assessment guidance and educational materials for clsi exempt. A unique iqcp should be implemented for each separate identification method or system. Iqcp stands for individualized. Find resources, examples and tips for evaluating. Quality control plan (iqcp) for your unique testing environment and your patients. Learn how to develop an iqcp. The cornerstone of iqcp is identifying, evaluating, and controlling potential sources of error relevant to the individual laboratory. Analyze the process of creating an iqcp’s step by step, using iqcp examples 3. Evaluate the impact of iqcp on quality control. A distinguished group of scientific organizations has revised the microbiology individualized quality control plan (iqcp) templates and created the first such template for. To facilitate completion of a risk assessment (ra) and adherence with the individualized quality control plan (iqcp) option, abbott point of care provides a guide to aid. Laboratories should. To facilitate completion of a risk assessment (ra) and adherence with the individualized quality control plan (iqcp) option, abbott point of care provides a guide to aid. Analyze the process of creating an iqcp’s step by step, using iqcp examples 3. This powerpoint presentation covers the cap checklist requirements,. Performing a risk assessment will facilitate the. Learn about the individualized. Quality control plan (iqcp) for your unique testing environment and your patients. Download templates, risk assessment guidance and educational materials for clsi exempt. Learn how to create an iqcp for your laboratory's testing process, based on risk assessment, quality control plan and quality assessment. Evaluate the impact of iqcp on quality control. Find iqcp resources and documentation for thermo fisher. Quality control plan (iqcp) for your unique testing environment and your patients. Learn how to apply individualized quality control plan (iqcp) for microbiology tests that are not performed each day of patient testing. This powerpoint presentation covers the cap checklist requirements,. A distinguished group of scientific organizations has revised the microbiology individualized quality control plan (iqcp) templates and created the. Find resources, examples and tips for evaluating. The cornerstone of iqcp is identifying, evaluating, and controlling potential sources of error relevant to the individual laboratory. Find iqcp resources and documentation for thermo fisher scientific microbiology products. Evaluate the impact of iqcp on quality control. Laboratories should modify this template and examples to support their practice. A unique iqcp should be implemented for each separate identification method or system. Find iqcp resources and documentation for thermo fisher scientific microbiology products. Iqcp stands for individualized quality control plan and is the alternative clia quality control (qc) option that will provide for equivalent quality testing to meet the clia regulations for. Learn how to apply individualized quality control. See a sample iqcp templa… Learn how to apply individualized quality control plan (iqcp) for microbiology tests that are not performed each day of patient testing. Iqcp permits the laboratory to customize its qc plan according to test method and use, environment, and personnel competency while providing for equivalent quality testing. Learn how to create an iqcp for your laboratory's. Find resources, examples and tips for evaluating. The document provides a template for implementing an individualized quality control plan (iqcp) for commercially prepared microbiological media, emphasizing the need for laboratories to. Laboratories should modify this template and examples to support their practice. A unique iqcp should be implemented for each separate identification method or system. The iqcp option offers your laboratory flexibility for meeting regulatory qc requirements appropriate for. Download templates, risk assessment guidance and educational materials for clsi exempt. Analyze the process of creating an iqcp’s step by step, using iqcp examples 3. See a sample iqcp templa… Performing a risk assessment will facilitate the. Learn how to create an iqcp for your laboratory's testing process, based on risk assessment, quality control plan and quality assessment. Learn how to develop an iqcp. A distinguished group of scientific organizations has revised the microbiology individualized quality control plan (iqcp) templates and created the first such template for. Evaluate the impact of iqcp on quality control. Quality control plan (iqcp) for your unique testing environment and your patients. Identify components that should be included in an iqcp 2. Learn how to apply individualized quality control plan (iqcp) for microbiology tests that are not performed each day of patient testing.Example IQCP for POC chemistry system Westgard
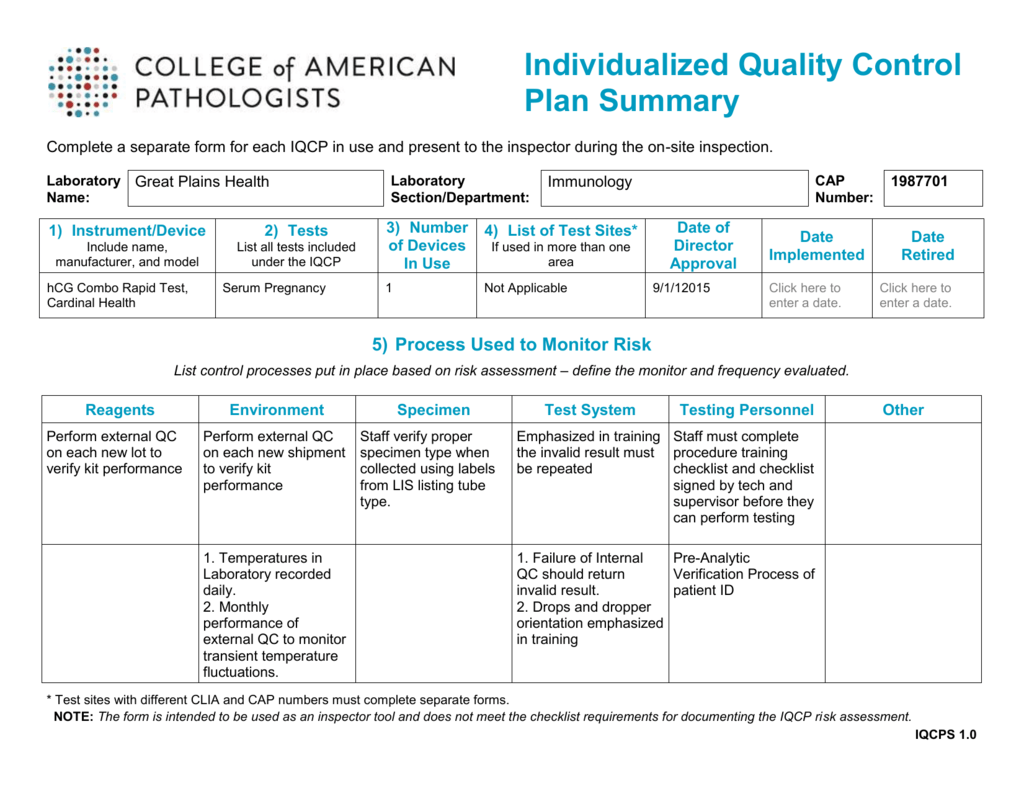
Individualized Quality Control Plan Summary
Individualized Quality Control Plan (IQCP)
IQCP Guidelines and Template for Getting Started Linda C
Develop Compliant IQCPs MarchApril 2015 MedicalLab Management Magazine
IQCP GUIDELINES and TEMPLATE FOR GETTING STARTED Doc Template pdfFiller
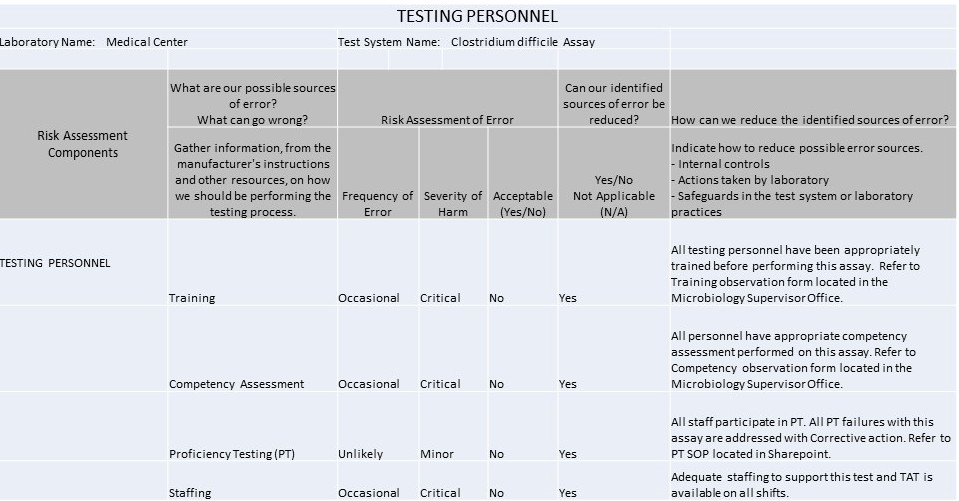
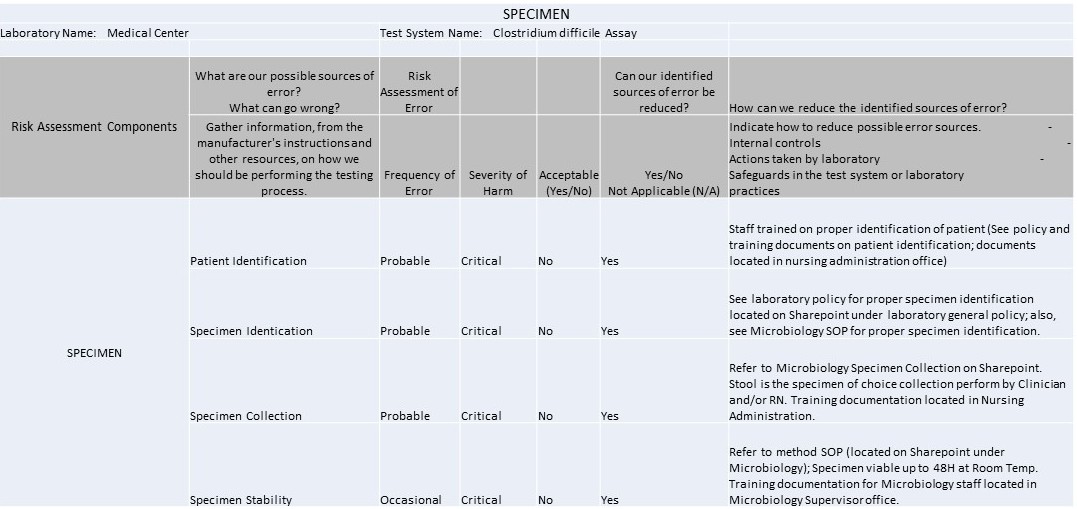
Example IQCP for C. Difficile Westgard QC
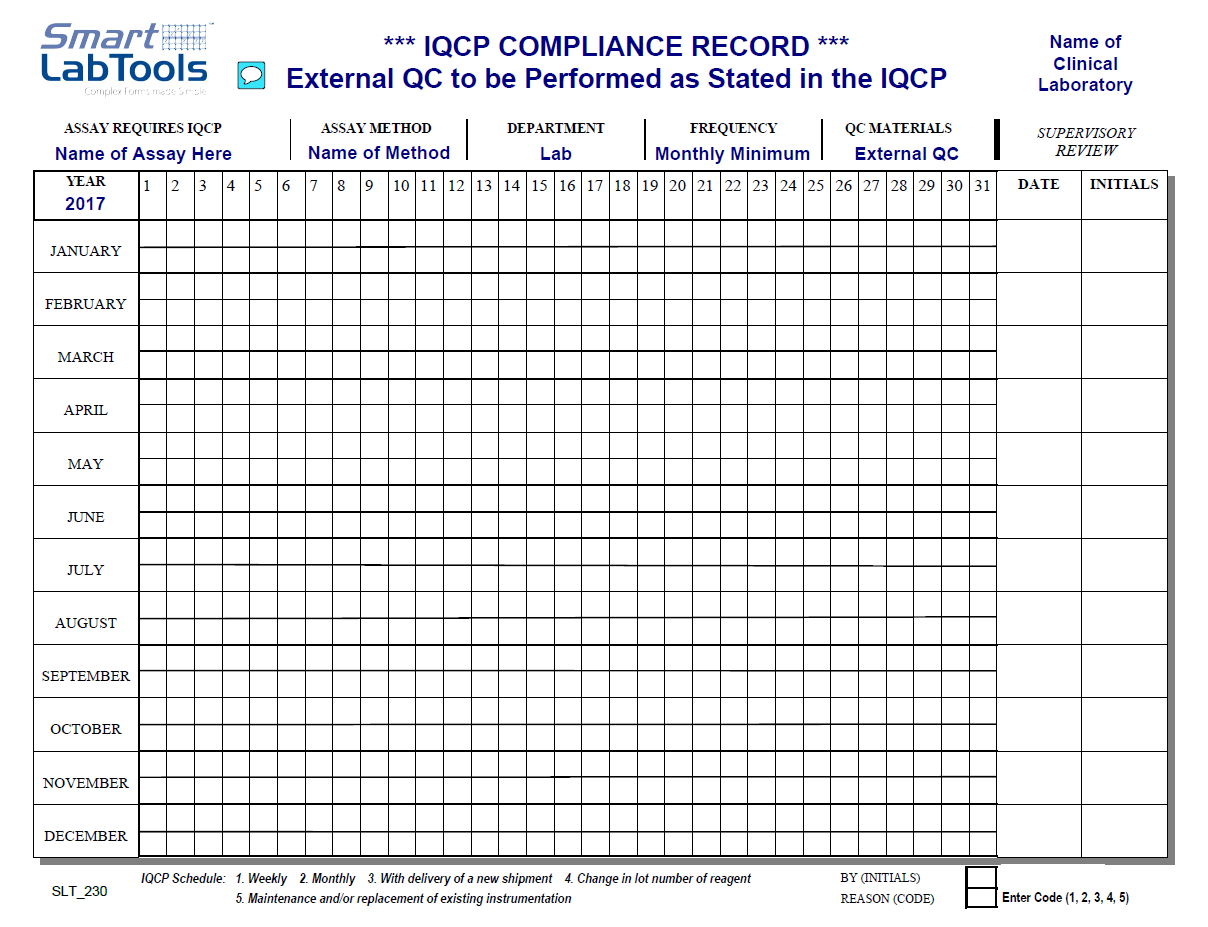
SmartLabTools SLT_IQCP Review Forms
Example IQCP for C. Difficile Westgard QC
Individualized Quality Control Plan (IQCP) Is It Value Doc
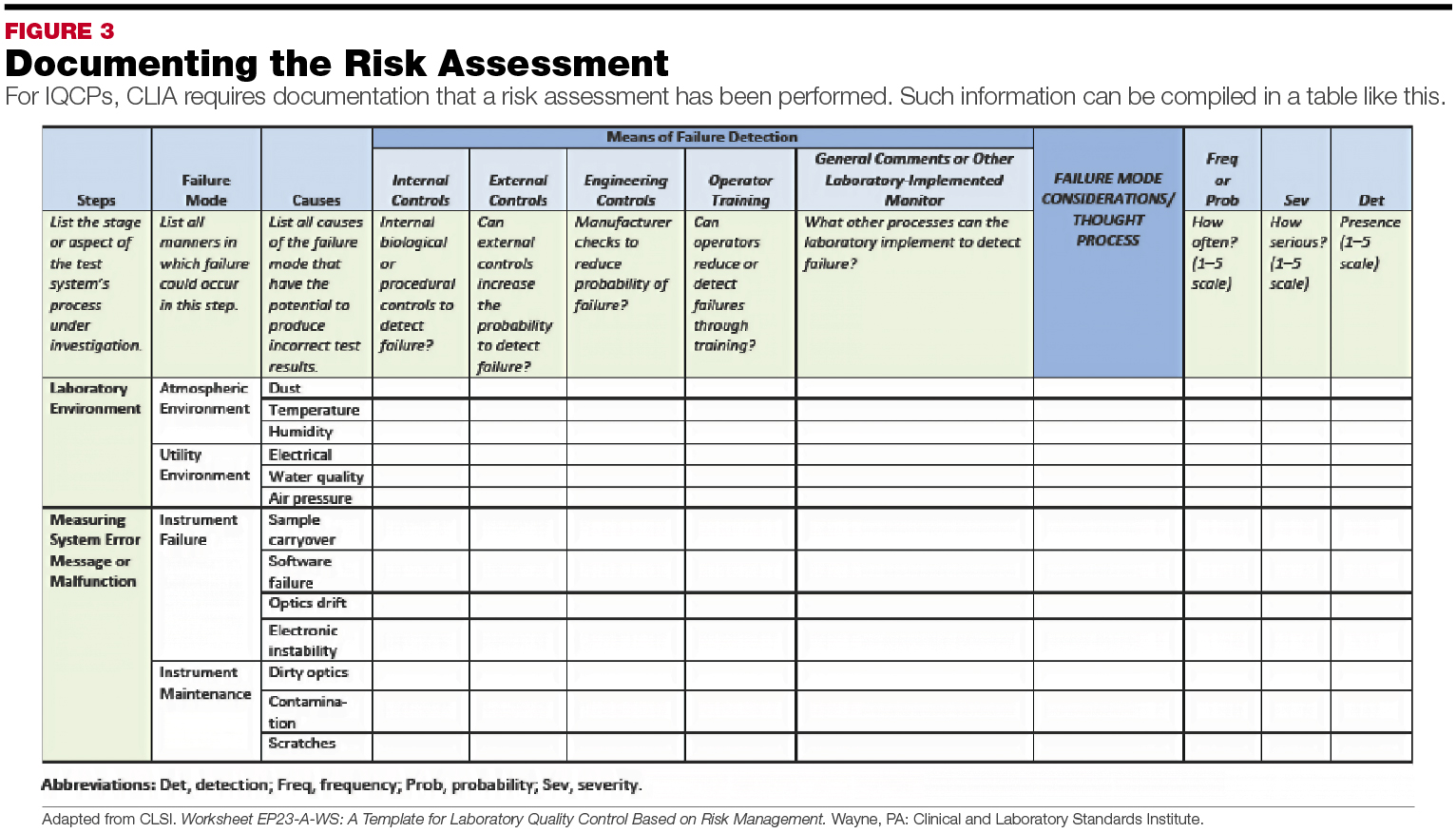
The Cornerstone Of Iqcp Is Identifying, Evaluating, And Controlling Potential Sources Of Error Relevant To The Individual Laboratory.
Iqcp Permits The Laboratory To Customize Its Qc Plan According To Test Method And Use, Environment, And Personnel Competency While Providing For Equivalent Quality Testing.
The Individualized Quality Control Plan (Iqcp) Option Offers The Laboratory Flexibility For Meeting Regulatory Qc Requirements.
To Facilitate Completion Of A Risk Assessment (Ra) And Adherence With The Individualized Quality Control Plan (Iqcp) Option, Abbott Point Of Care Provides A Guide To Aid.
Related Post: