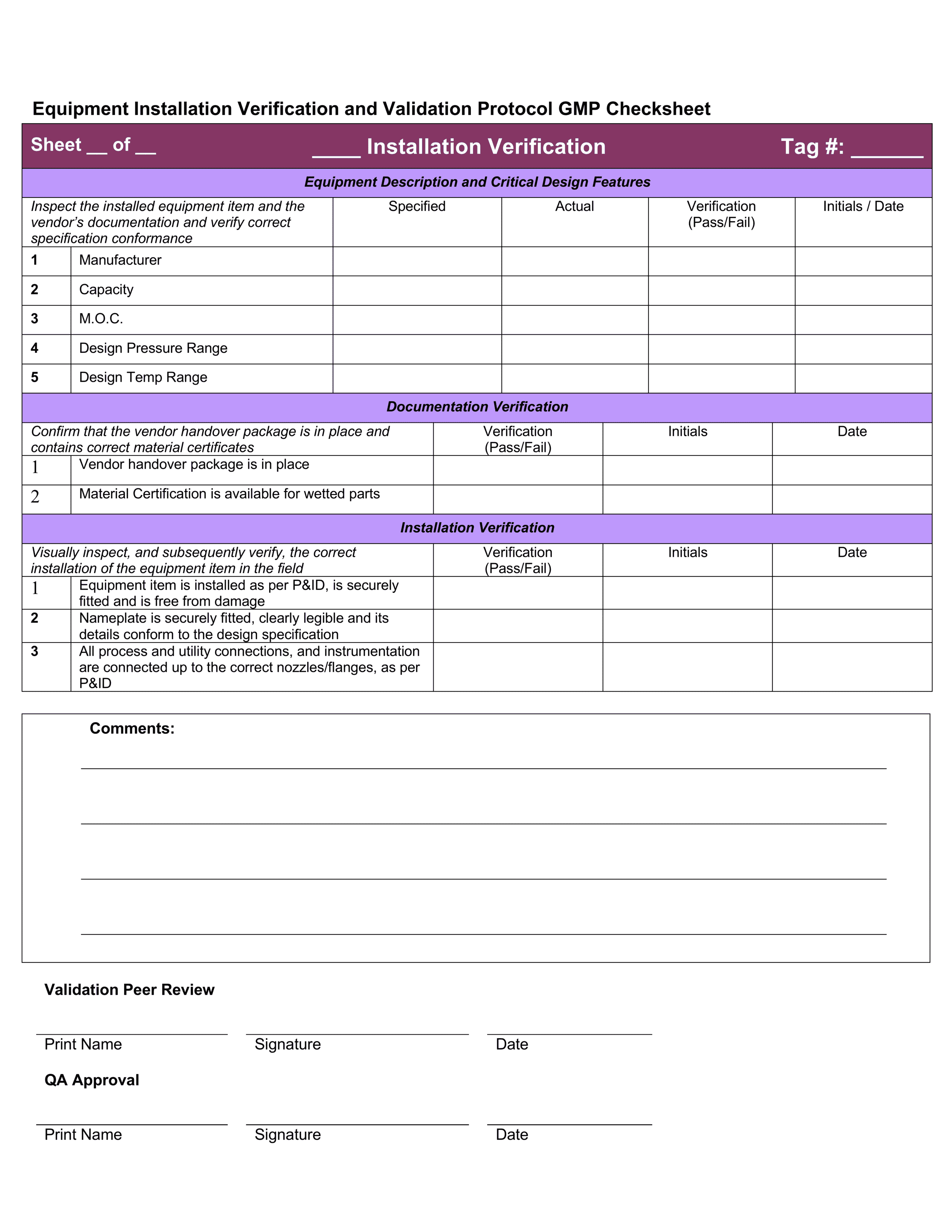
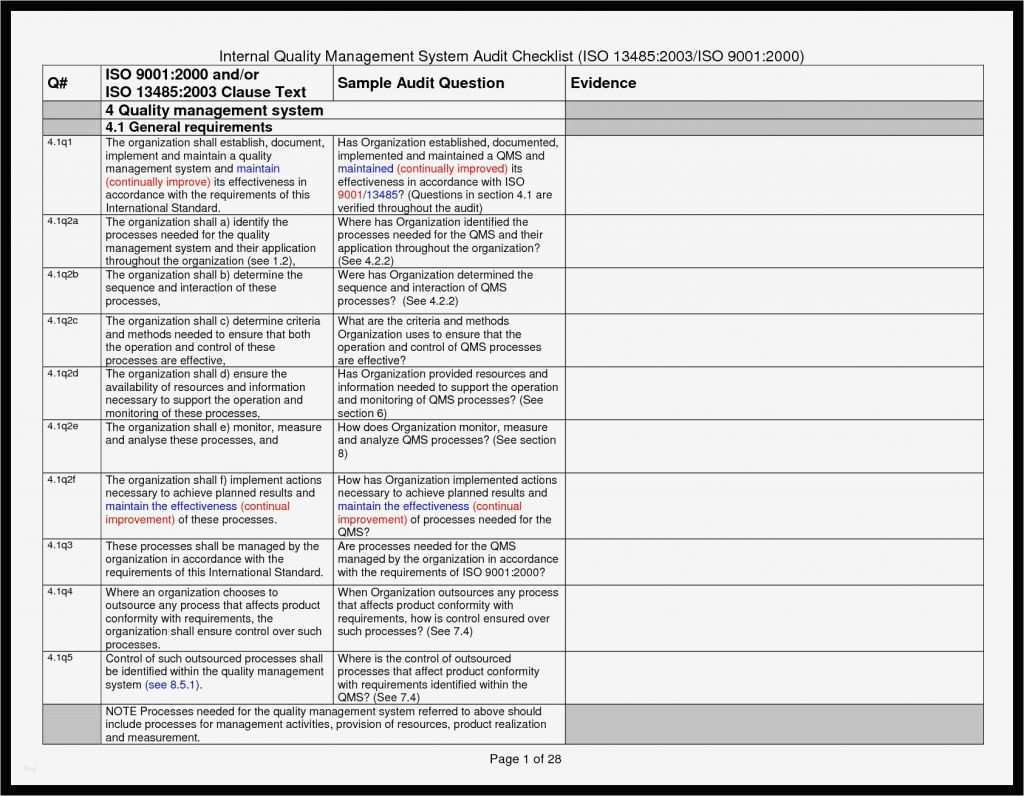
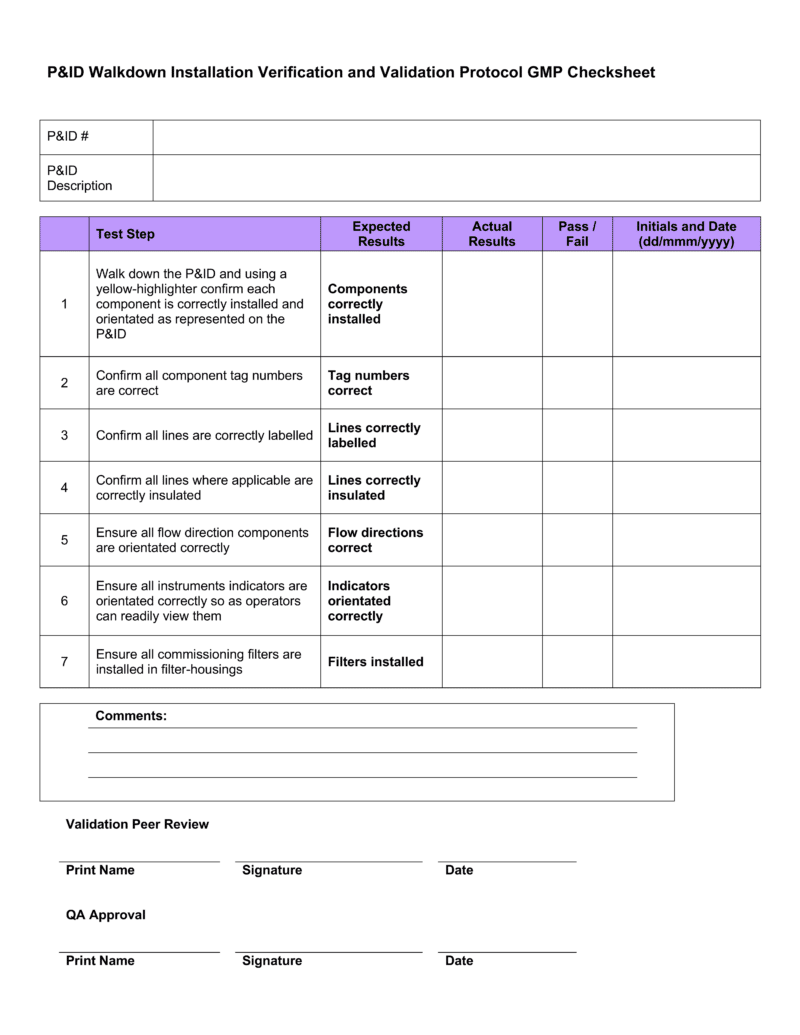
Iq Oq Pq Template
Iq Oq Pq Template - The combined qualification has been carefully designed. The intent of this dq/iq/oq/pq protocol is to define and assure the implementation of the organizational practices, standards, methods, and documentation conventions to be used for. Learn what iq, oq, pq are and how to perform them in pharmaceutical, medical devices, and clinical industries. This is a combination of the iq, oq, and pq. This sop applies to all iq, oq, and pq activities performed on pharmaceutical manufacturing equipment, systems, and processes. Learn about iq oq pq validation processes, iq oq pq examples, and essential iq oq templates to ensure quality in your manufacturing operations. It covers the documentation of iq/oq/pq. Performance qualification (pq) demonstrate the process will consistently produce acceptable product under normal operating conditions. The objective of this protocol is to define the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the [insert system name and plant. This template is suitable for authoring the tests of either user requirements (pq) or functional requirements (oq) template sections include: You can use this for a full qualification, add or remove any sections as you require. Learn what iq, oq, pq are and how to perform them in pharmaceutical, medical devices, and clinical industries. The intent of this dq/iq/oq/pq protocol is to define and assure the implementation of the organizational practices, standards, methods, and documentation conventions to be used for. It also produces the thorough audit trail needed to meet all. This is a combination of the iq, oq, and pq. Learn about iq oq pq validation processes, iq oq pq examples, and essential iq oq templates to ensure quality in your manufacturing operations. Does on average, reduce protocol authoring, and execution approval times by 40%. This template is suitable for authoring the tests of either user requirements (pq) or functional requirements (oq) template sections include: Iq oq pq medical devices process validation is conducted to ensure consistent delivery of quality products meeting its predetermined specifications and quality characteristics. It covers the documentation of iq/oq/pq. This is a combination of the iq, oq, and pq. Learn about iq oq pq validation processes, iq oq pq examples, and essential iq oq templates to ensure quality in your manufacturing operations. The intent of this dq/iq/oq/pq protocol is to define and assure the implementation of the organizational practices, standards, methods, and documentation conventions to be used for. Iq. Things to consider… • approved procedures and. This is a combination of the iq, oq, and pq. This sop applies to all iq, oq, and pq activities performed on pharmaceutical manufacturing equipment, systems, and processes. Learn what iq, oq, pq are and how to perform them in pharmaceutical, medical devices, and clinical industries. The objective of this protocol is to. The combined qualification has been carefully designed. Iq oq pq medical devices process validation is conducted to ensure consistent delivery of quality products meeting its predetermined specifications and quality characteristics. This sop applies to all iq, oq, and pq activities performed on pharmaceutical manufacturing equipment, systems, and processes. Learn what iq, oq, pq are and how to perform them in. This is a combination of the iq, oq, and pq. Learn what iq, oq, pq are and how to perform them in pharmaceutical, medical devices, and clinical industries. Does on average, reduce protocol authoring, and execution approval times by 40%. Iq oq pq medical devices process validation is conducted to ensure consistent delivery of quality products meeting its predetermined specifications. Does on average, reduce protocol authoring, and execution approval times by 40%. Learn about iq oq pq validation processes, iq oq pq examples, and essential iq oq templates to ensure quality in your manufacturing operations. This is a combination of the iq, oq, and pq. This template is suitable for authoring the tests of either user requirements (pq) or functional. You can use this for a full qualification, add or remove any sections as you require. Learn about iq oq pq validation processes, iq oq pq examples, and essential iq oq templates to ensure quality in your manufacturing operations. It covers the documentation of iq/oq/pq. The combined qualification has been carefully designed. Things to consider… • approved procedures and. It covers the documentation of iq/oq/pq. The combined qualification has been carefully designed. Learn about iq oq pq validation processes, iq oq pq examples, and essential iq oq templates to ensure quality in your manufacturing operations. This template is suitable for authoring the tests of either user requirements (pq) or functional requirements (oq) template sections include: The intent of this. It covers the documentation of iq/oq/pq. Things to consider… • approved procedures and. The objective of this protocol is to define the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the [insert system name and plant. The combined qualification has been carefully designed. It also produces the thorough audit trail needed to meet all. Iq oq pq medical devices process validation is conducted to ensure consistent delivery of quality products meeting its predetermined specifications and quality characteristics. Does on average, reduce protocol authoring, and execution approval times by 40%. Learn about iq oq pq validation processes, iq oq pq examples, and essential iq oq templates to ensure quality in your manufacturing operations. The intent. Iq oq pq medical devices process validation is conducted to ensure consistent delivery of quality products meeting its predetermined specifications and quality characteristics. You can use this for a full qualification, add or remove any sections as you require. This template is suitable for authoring the tests of either user requirements (pq) or functional requirements (oq) template sections include: This. Learn what iq, oq, pq are and how to perform them in pharmaceutical, medical devices, and clinical industries. Find out the best practices, challenges, and tips for. It also produces the thorough audit trail needed to meet all. This sop applies to all iq, oq, and pq activities performed on pharmaceutical manufacturing equipment, systems, and processes. Performance qualification (pq) demonstrate the process will consistently produce acceptable product under normal operating conditions. This is a combination of the iq, oq, and pq. The objective of this protocol is to define the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the [insert system name and plant. Learn about iq oq pq validation processes, iq oq pq examples, and essential iq oq templates to ensure quality in your manufacturing operations. You can use this for a full qualification, add or remove any sections as you require. It covers the documentation of iq/oq/pq. Things to consider… • approved procedures and. The intent of this dq/iq/oq/pq protocol is to define and assure the implementation of the organizational practices, standards, methods, and documentation conventions to be used for.Iq Oq Pq Template
Six Sigma Validation Process IQ Installation Qualification OQ
Iq Oq Pq Vorlage Schön Iopq Freezer Validation Template Sample by
Iq Oq Pq Templates Download 4 Free Professional Templates Images
Iq Oq Pq Templates
What is IQ, OQ, PQ? [Quick Guide to Process Validation]
Iq Oq Pq Templates
Iq Oq Pq Vorlage Schön 10 New Iq Oq Pq Validation Templates
Iq Oq Pq Template
Iq Oq Pq Templates
The Combined Qualification Has Been Carefully Designed.
Iq Oq Pq Medical Devices Process Validation Is Conducted To Ensure Consistent Delivery Of Quality Products Meeting Its Predetermined Specifications And Quality Characteristics.
Does On Average, Reduce Protocol Authoring, And Execution Approval Times By 40%.
This Template Is Suitable For Authoring The Tests Of Either User Requirements (Pq) Or Functional Requirements (Oq) Template Sections Include:
Related Post:



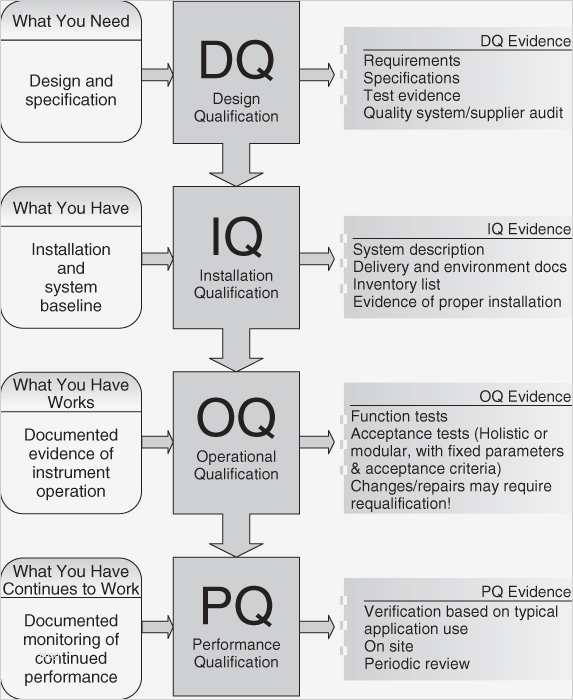
![What is IQ, OQ, PQ? [Quick Guide to Process Validation]](https://blog.greenlight.guru/hubfs/IQ OQ PQ.png)