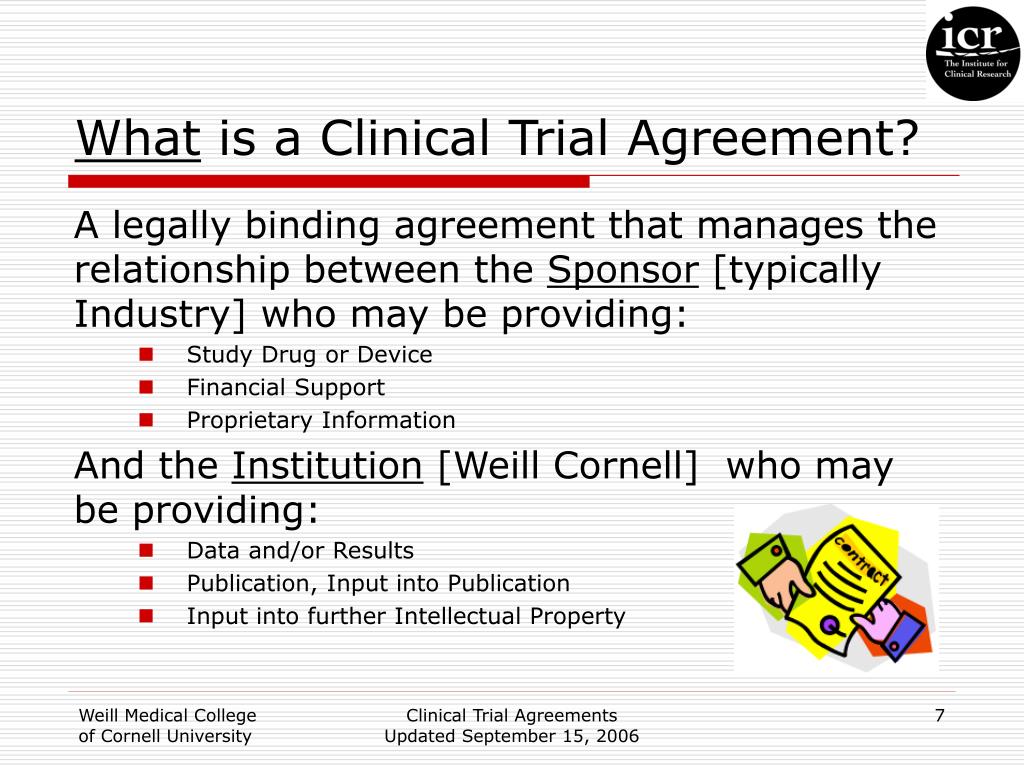
Clinical Trial Contract Template
Clinical Trial Contract Template - This agreement is entered into on _______________________by and between ____________________________________________________________________. Welcome to global health trials' tools and templates library. • the regents of the university of california on behalf of its. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many new ones. “cgcp” means current good clinical practices as established pursuant to the regulations and guidelines of the regulatory authority or regulatory authorities having jurisdiction. This early feasibility study (“efs”) master clinical trial agreement template is provided by the medical device. Instantly download clinical trial agreement template sample & example in pdf, microsoft word (doc), google docs, and apple pages format. This clinical trial agreement is between a university and a sponsor. With a robust clinical trial agreement template in place and a cms like juro, you can initiate contracts within seconds. See notices of special interest associated with this funding opportunity. Sponsor is a pharmaceutical company involved in the research, development, manufacture and sale of medicines for use in humans and wishes to contract with the institution and the. Phone, email & live helpmobile & desktoptrusted legal forms Efs master clinical study agreement important note: With a robust clinical trial agreement template in place and a cms like juro, you can initiate contracts within seconds. Make, sign & save a customized clinical trial agreement with rocket lawyer. This early feasibility study (“efs”) master clinical trial agreement template is provided by the medical device innovation consortium (“mdic”) as an educational tool. This early feasibility study (“efs”) master clinical trial agreement template is provided by the medical device. See notices of special interest associated with this funding opportunity. The clinical trial agreement (“agreement”) is made as of the later of the signatures below (“ effective date ”) by and between: • the regents of the university of california on behalf of its. “cgcp” means current good clinical practices as established pursuant to the regulations and guidelines of the regulatory authority or regulatory authorities having jurisdiction. Need a clinical trial agreement that details about legal aspects and party obligations? Use this free clinical trial agreement template to manage the relationship between the sponsor and the institution. This agreement is entered into on _______________________by. This legal template involves a simple contract between a. With a robust clinical trial agreement template in place and a cms like juro, you can initiate contracts within seconds. Accelerated clinical trial agreement this accelerated clinical trial (acta) agreement (“agreement”) is made as of this _____ day of _____ [month] , _ _____ [year] (the. This early feasibility study (“efs”). Instantly download clinical trial agreement template sample & example in pdf, microsoft word (doc), google docs, and apple pages format. Welcome to global health trials' tools and templates library. Up to 24% cash back set terms for clinical trial product testing. Use this free clinical trial agreement template to manage the relationship between the sponsor and the institution. Accelerated clinical. Up to 24% cash back set terms for clinical trial product testing. See notices of special interest associated with this funding opportunity. This agreement is entered into on _______________________by and between ____________________________________________________________________. Need a clinical trial agreement that details about legal aspects and party obligations? Please note that this page has been updated for 2015 following a quality check and. Use this free clinical trial agreement template to manage the relationship between the sponsor and the institution. This legal template involves a simple contract between a. Agreement underscores norgine’s commitment to bring transformative therapies to patients in need in these key strategic territories. This agreement is entered into on _______________________by and between ____________________________________________________________________. Welcome to global health trials' tools and. Sponsor is a pharmaceutical company involved in the research, development, manufacture and sale of medicines for use in humans and wishes to contract with the institution and the. This legal template involves a simple contract between a. This clinical trial agreement is between a university and a sponsor. Use this free clinical trial agreement template to manage the relationship between. • the regents of the university of california on behalf of its. Efs master clinical study agreement important note: With a robust clinical trial agreement template in place and a cms like juro, you can initiate contracts within seconds. This legal template involves a simple contract between a. Up to 24% cash back set terms for clinical trial product testing. Make, sign & save a customized clinical trial agreement with rocket lawyer. See notices of special interest associated with this funding opportunity. Agreement underscores norgine’s commitment to bring transformative therapies to patients in need in these key strategic territories. Use this free clinical trial agreement template to manage the relationship between the sponsor and the institution. • the regents of. Up to 24% cash back set terms for clinical trial product testing. Sponsor is a pharmaceutical company involved in the research, development, manufacture and sale of medicines for use in humans and wishes to contract with the institution and the. Accelerated clinical trial agreement this accelerated clinical trial (acta) agreement (“agreement”) is made as of this _____ day of _____. Efs master clinical study agreement important note: “cgcp” means current good clinical practices as established pursuant to the regulations and guidelines of the regulatory authority or regulatory authorities having jurisdiction. • the regents of the university of california on behalf of its. Sponsor is a pharmaceutical company involved in the research, development, manufacture and sale of medicines for use in. Optimize your job with our vast range of template samples. This clinical trial agreement is between a university and a sponsor. Up to 24% cash back set terms for clinical trial product testing. This agreement is entered into on _______________________by and between ____________________________________________________________________. Agreement underscores norgine’s commitment to bring transformative therapies to patients in need in these key strategic territories. This legal template involves a simple contract between a. The clinical trial agreement (“agreement”) is made as of the later of the signatures below (“ effective date ”) by and between: The templates are automatically populated using a straightforward. This early feasibility study (“efs”) master clinical trial agreement template is provided by the medical device innovation consortium (“mdic”) as an educational tool. See notices of special interest associated with this funding opportunity. This early feasibility study (“efs”) master clinical trial agreement template is provided by the medical device. “cgcp” means current good clinical practices as established pursuant to the regulations and guidelines of the regulatory authority or regulatory authorities having jurisdiction. Efs master clinical study agreement important note: With a robust clinical trial agreement template in place and a cms like juro, you can initiate contracts within seconds. Welcome to global health trials' tools and templates library. Sponsor is a pharmaceutical company involved in the research, development, manufacture and sale of medicines for use in humans and wishes to contract with the institution and the.Free Clinical Trial Agreement Free to Print, Save & Download
Template clinical trial agreement available for investigator Doc
CLINICAL TRIALS CONTRACT Doc Template pdfFiller
Template clinical trial agreement available forHomeThe Central
OHSU Clinical Trial Agreement Template
Clinical Trial Agreement Template
investigator agreement clinical trial Doc Template pdfFiller
Clinical Trial Agreement SEC.gov Doc Template pdfFiller
english.ccmo.nllatestnewsTemplate clinical trial agreement available
Master Clinical Trial Agreement Template in Word, PDF, Google Docs
Use This Free Clinical Trial Agreement Template To Manage The Relationship Between The Sponsor And The Institution.
Need A Clinical Trial Agreement That Details About Legal Aspects And Party Obligations?
Clinical Trial Contracts Are Legally Binding Documents That Define The Rights And Responsibilities Of The Parties Involved In A Clinical Trial.
Please Note That This Page Has Been Updated For 2015 Following A Quality Check And Review Of The Templates, And Many New Ones.
Related Post: