Clinical Evaluation Plan Template
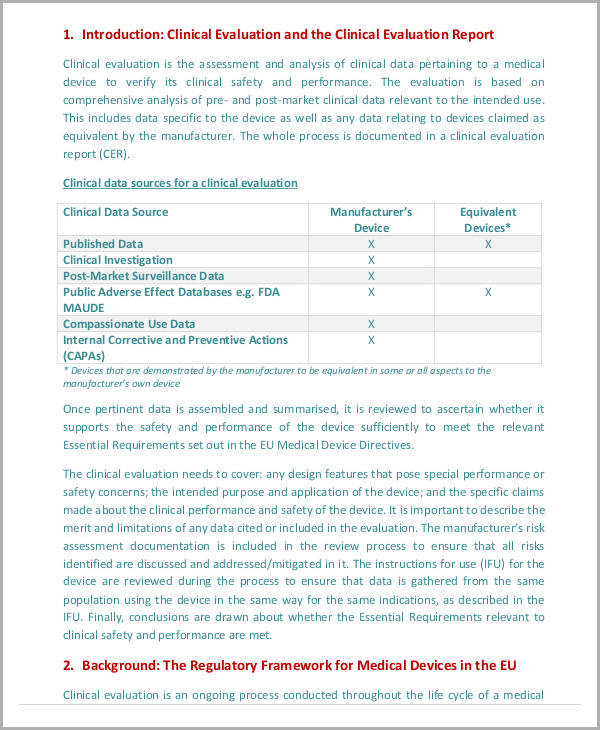
Clinical Evaluation Plan Template - What is a clinical evaluation plan? A clinical evaluation plan (cep) is an important technical document that sets out a clear plan for conducting a clinical evaluation of a medical device. Clinical evaluation plan is a road map for conducting the clinical evaluation process. Documenting a clinical evaluation plan. 의료기기의 안전성과 성능을 입증하기 위해 체계적인 임상 개발 계획(clinical development plan, cdp)의 수립은 필수적입니다. If you’re the person writing it, you should read them: In this article, we will explore the purpose and scope of clinical evaluation, key components of a clinical evaluation plan template, the impact of device classification on clinical. The clinical evaluation plan defines methods for creating and updating the clinical evaluation report. The clinical evaluation plan defines methods for creating and updating the clinical evaluation report. The following checklist provides an overview of possible documents and. A free template to help you get started with creating your medical device company's clinical evaluation standard operating procedure (sop) in accordance with eu mdr 2017/745. If you’re the person writing it, you should read them: Documenting a clinical evaluation plan. In this article, we will explore the purpose and scope of clinical evaluation, key components of a clinical evaluation plan template, the impact of device classification on clinical. The following checklist provides an overview of possible documents and. The clinical evaluation plan outlines the methods for developing and updating the clinical evaluation report. Clinical evaluation plan is a road map for conducting the clinical evaluation process. 의료기기의 안전성과 성능을 입증하기 위해 체계적인 임상 개발 계획(clinical development plan, cdp)의 수립은 필수적입니다. Unless specified in a program solicitation, all. A clinical evaluation plan (cep) is an important technical document that sets out a clear plan for conducting a clinical evaluation of a medical device. These are the guidance documents on clinical evaluation. 의료기기의 안전성과 성능을 입증하기 위해 체계적인 임상 개발 계획(clinical development plan, cdp)의 수립은 필수적입니다. A free template to help you get started with creating your medical device company's clinical evaluation standard operating procedure (sop) in accordance with eu mdr 2017/745. The clinical evaluation plan defines methods for creating and updating the clinical. If you’re the person writing it, you should read them: Deviations from nsf proposal preparation and submission requirements. The clinical evaluation plan outlines the methods for developing and updating the clinical evaluation report. Conformance with instructions for proposal preparation 1. The clinical evaluation plan is the foundation of the overall clinical evaluation process and. (cep) what is the purpose of a clinical evaluation plan? Employ the rules to develop objectives and. Clinical evaluation plan is a road map for conducting the clinical evaluation process. The clinical evaluation plan outlines the methods for developing and updating the clinical evaluation report. A clinical evaluation plan (cep) is an important technical document that sets out a clear. Unless specified in a program solicitation, all. Conformance with instructions for proposal preparation 1. Clinical evaluation plan is a road map for conducting the clinical evaluation process. It includes the scope, methodological, and systematic approach on how to proceed and reach a. Learn how to write a clinical evaluation plan for a medical device, including the scope, data sources, requirements,. The clinical evaluation plan defines methods for creating and updating the clinical evaluation report. The clinical evaluation plan outlines the methods for developing and updating the clinical evaluation report. (cep) what is the purpose of a clinical evaluation plan? Clinical evaluation plan is a road map for conducting the clinical evaluation process. The following checklist provides an overview of possible. Documenting a clinical evaluation plan. Learn how to write a clinical evaluation plan for a medical device, including the scope, data sources, requirements, and responsible personnel. (cep) what is the purpose of a clinical evaluation plan? If you’re the person writing it, you should read them: Employ the rules to develop objectives and. If you’re the person writing it, you should read them: The clinical evaluation plan defines methods for creating and updating the clinical evaluation report. What is a clinical evaluation plan? The clinical evaluation plan outlines the methods for developing and updating the clinical evaluation report. Find a detailed template and a proposal. Deviations from nsf proposal preparation and submission requirements. (cep) what is the purpose of a clinical evaluation plan? A clinical evaluation plan (cep) is an important technical document that sets out a clear plan for conducting a clinical evaluation of a medical device. Unless specified in a program solicitation, all. Employ the rules to develop objectives and. Learn how to write a clinical evaluation plan for a medical device, including the scope, data sources, requirements, and responsible personnel. The clinical evaluation plan defines methods for creating and updating the clinical evaluation report. A free template to help you get started with creating your medical device company's clinical evaluation standard operating procedure (sop) in accordance with eu mdr. If you’re the person writing it, you should read them: The following checklist provides an overview of possible documents and. The clinical evaluation plan outlines the methods for developing and updating the clinical evaluation report. Find a detailed template and a proposal. These are the guidance documents on clinical evaluation. According to the regulation (eu) 2017/745, article 61 and annex xiv, the evaluation of the clinical performance and safety as well as the clinical benefit must be based. Attain complete autonomy through a proven 11 section cep framework. Conformance with instructions for proposal preparation 1. Deviations from nsf proposal preparation and submission requirements. Find a detailed template and a proposal. The clinical evaluation plan defines methods for creating and updating the clinical evaluation report. (cep) what is the purpose of a clinical evaluation plan? Unless specified in a program solicitation, all. A free template to help you get started with creating your medical device company's clinical evaluation standard operating procedure (sop) in accordance with eu mdr 2017/745. If you’re the person writing it, you should read them: Learn how to write a clinical evaluation plan for a medical device, including the scope, data sources, requirements, and responsible personnel. The clinical evaluation plan defines methods for creating and updating the clinical evaluation report. The clinical evaluation plan defines methods for creating and updating the clinical evaluation report. Clinical evaluation plan is a document that has all necessary elements for planning the clinical evaluation process required by mdr and additional guidelines. 의료기기의 안전성과 성능을 입증하기 위해 체계적인 임상 개발 계획(clinical development plan, cdp)의 수립은 필수적입니다. In this article, we will explore the purpose and scope of clinical evaluation, key components of a clinical evaluation plan template, the impact of device classification on clinical.Clinical Evaluation Report Template Mdr
Clinical Evaluation Plan Template
Clinical Evaluation Plan/Report Fill and Sign Printable Template
Clinical Evaluation Plan (CEP) Template Clinical Study Templates
Clinical Evaluation Plan Template
Clinical Evaluation Plan Template 20202022 Fill and Sign Printable
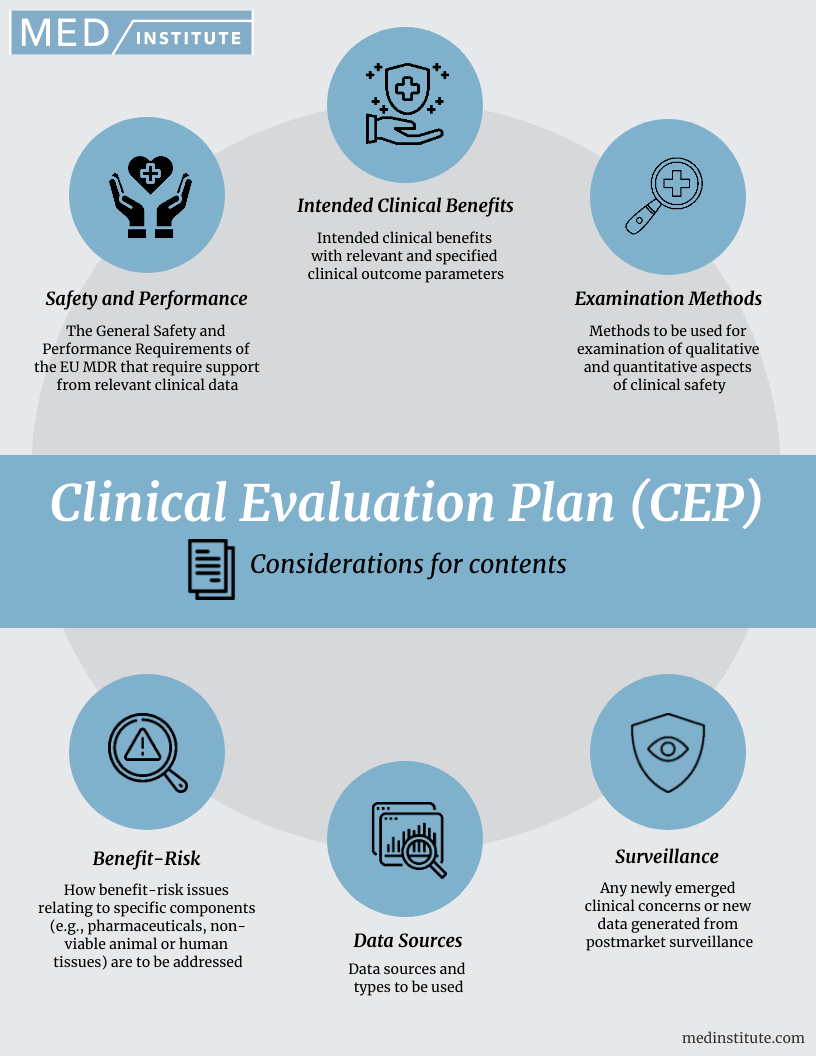
Clinical Evaluation Plan (CEP) 6 Content Considerations MED Institute
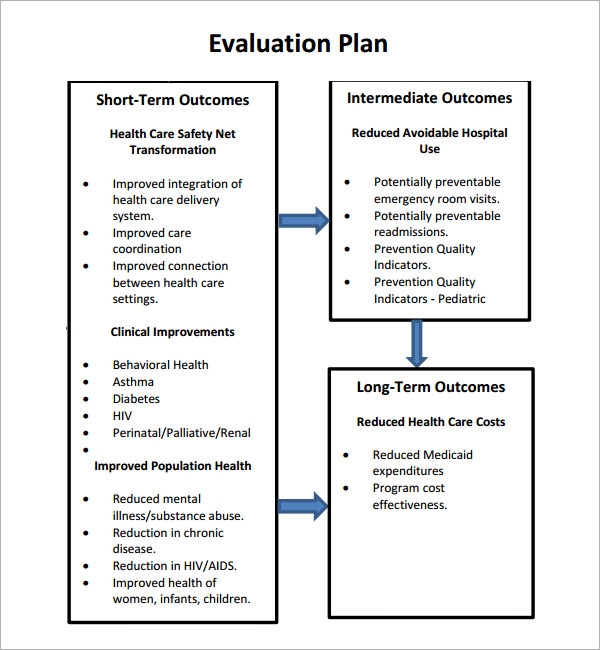
FREE 8+ Evaluation Plan Templates in MS Word PDF
Clinical Evaluation Plan Template
Clinical Evaluation Plan Template
Documenting A Clinical Evaluation Plan.
Download A Free Sample Of Our Cep Template To See How It Works.
The Clinical Evaluation Plan Is The Foundation Of The Overall Clinical Evaluation Process And.
A Clinical Evaluation Plan (Cep) Is An Important Technical Document That Sets Out A Clear Plan For Conducting A Clinical Evaluation Of A Medical Device.
Related Post: